What’s happening
The monkeypox vaccine is available to people at higher risk of getting the disease. Because supply has been so limited, health officials authorized a different vaccination method, which requires a lower dose of vaccine.
Why it matters
Vaccination is an important tool to slow the monkeypox outbreak happening in the US and other countries. Access to vaccines is crucial for people most at risk.
What it means for you
Some men who have sex with men are eligible for the vaccine, as are other people who may’ve been exposed to monkeypox.
The US Food and Drug Administration on Tuesday greenlighted a different monkeypox vaccination method, which requires a much smaller dose, potentially increasing the US supply of Jynneos up to five times.
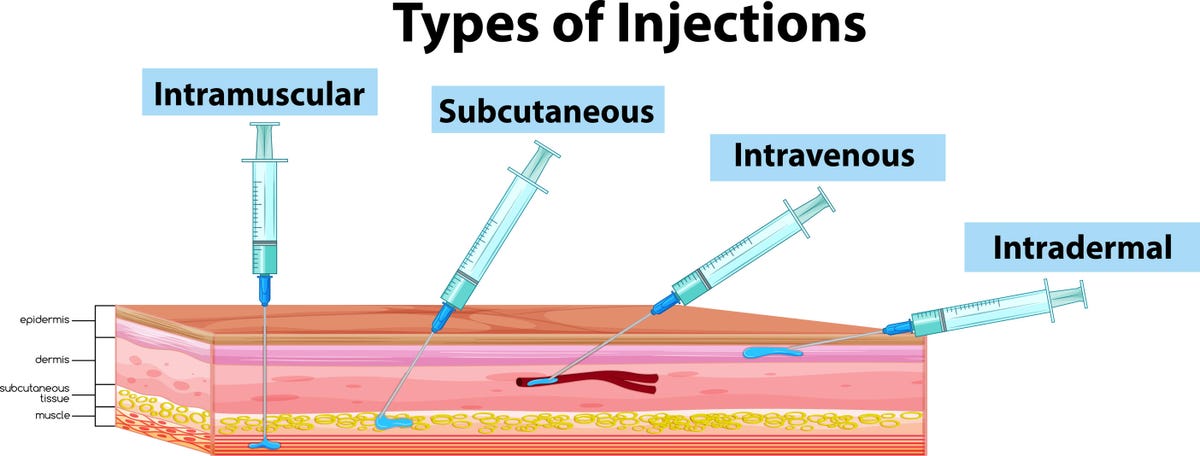
Jynneos is the monkeypox vaccine currently available to people at higher risk of the disease. With the FDA’s decision, it can now be given as an intradermal vaccination as opposed to only a subcutaneous injection. With intradermal, the injection goes under a top layer of skin. With subcutaneous, the shot is administered deeper, under a layer of fatty tissue.
Also called “dose-sparing,” intradermal types of injections aren’t new to health care. But the change in medical guidance on how the monkeypox vaccine can be administered reflects a shortage of Jynneos that’s plagued the vaccine rollout since it began.
“In recent weeks the monkeypox virus has continued to spread at a rate that has made it clear our current vaccine supply will not meet the current demand,” FDA Commissioner Robert M. Califf said in a press release. “By increasing the number of available doses, more individuals who want to be vaccinated against monkeypox will now have the opportunity to do so.”
The US Department of Health and Human Services has been shipping out vaccine doses to states based on need and supply. As of Aug. 8, the agency said it’s distributed more than 600,000 doses to cities and states throughout the US. About 3.5 million doses of the vaccine are needed to fight the outbreak, according to an estimate reported by The New York Times.
The federal government has promised that 6.9 million doses total will be available by mid-2023, but some critics have warned that the public health response to monkeypox has been much too slow and that by the time those doses are able to get into people’s arms, it may be too late to stop the disease from spreading widely in the US.
“We’re sort of playing catch-up,” said Dr. Amesh Adalja, an infectious-disease expert and senior scholar at the Johns Hopkins Center for Health Security.
In areas with a lot of cases, like San Francisco, New York City and Washington, DC, people who are eligible for the vaccine are getting only the first dose of the two-dose series. Officials say this will still offer protection against monkeypox and that it’s in line with the response in other countries, including Canada and the UK.
Here’s what we know about the Jynneos monkeypox vaccine and who’s eligible for it. Also, here’s what to know about ACAM2000, the older generation vaccine that also works against monkeypox.
Read more: Monkeypox Explained: Symptoms, Treatment and How It Spreads
What is intradermal vaccination?
Intradermal vaccination is when the vaccine goes under a more shallow layer of skin, typically on the inner side of the forearm. This should produce a “noticeable pale elevation of the skin,” according to the US Centers for Disease Control and Prevention. Jynneos should still be given in two doses, four weeks apart, with the new method.
Most vaccines we get these days go either into the muscle in our arm (intramuscular injection) or into the fatty tissue under our skin (subcutaneous injection), like Jynneos has been given so far. Subcutaneous injection of Jynneos is the only authorized method of vaccination for people younger than 18, per the FDA.
Intradermal vaccination for monkeypox requires less vaccine to spur an immune response, health officials say. When the FDA authorized the new vaccine method, the agency referenced a study published in 2015 that found that a smallpox vaccine given intradermally gave a similar immune response in people compared to the vaccine given subcutaneously.
It isn’t clear at this point how many cities or vaccination sites will adopt, or have already adopted, the new method of vaccination. It’ll likely require a little additional training on the health care provider’s part.

Why is there a monkeypox vaccine supply problem?
The US has a supply of vaccines that work against monkeypox not because it anticipated a monkeypox outbreak, but in case of another smallpox outbreak, which typically causes much more severe disease. (More on this below.) However, the supply of Jynneos, the newer vaccine specifically approved to prevent monkeypox, has been in particularly short supply.
Officials who spoke to The New York Times said the supply issue is partly because the government waited too long to ask Bavarian Nordic, which makes Jynneos, to bottle and fill the vaccine order the US had already purchased.
Another report by the Times alleges that the US national stockpile of monkeypox and smallpox vaccines dwindled because the government never replaced the expired doses and instead put money into technology that would extend their shelf life. Part of the reason for this was that the government created the stockpile not for monkeypox but for smallpox, which is a more contagious and often more serious disease officials fear could be used in biological warfare against the US.
The US Department of Health and Human Services didn’t respond to a request for comment on the reports.
Who’s eligible for the vaccine and how do I get one?
Exact criteria for who should get a monkeypox vaccine depend on the city or state people live in and how widespread the outbreak is there, but men who have sex with men and who have had multiple or anonymous sexual partners in the last two weeks are eligible in cities like New York. That’s because gay and bisexual men are currently at higher risk in the outbreak, though anyone with close contact to monkeypox can get the disease.
According to the CDC, you meet the criteria for a monkeypox vaccine if:
- You’re a contact of someone who has monkeypox or you were identified as possibly exposed via contact tracing.
- You had a sexual partner within the last two weeks who has monkeypox.
- You’ve had multiple sexual partners in the last two weeks in an area with a high number of monkeypox cases.
- You are a lab or health care worker who’s around orthopox viruses, including in animals.
If you think you qualify for a vaccine or were exposed to monkeypox, contact your local health department or doctor’s office to find an appointment in your area. You can also book an appointment for the vaccine online, a process that’ll walk you through the eligibility criteria. Here is New York City’s vaccine appointment website. You can make an appointment and find a vaccine in San Francisco by calling one of the city’s clinics or visiting its drop-in location.
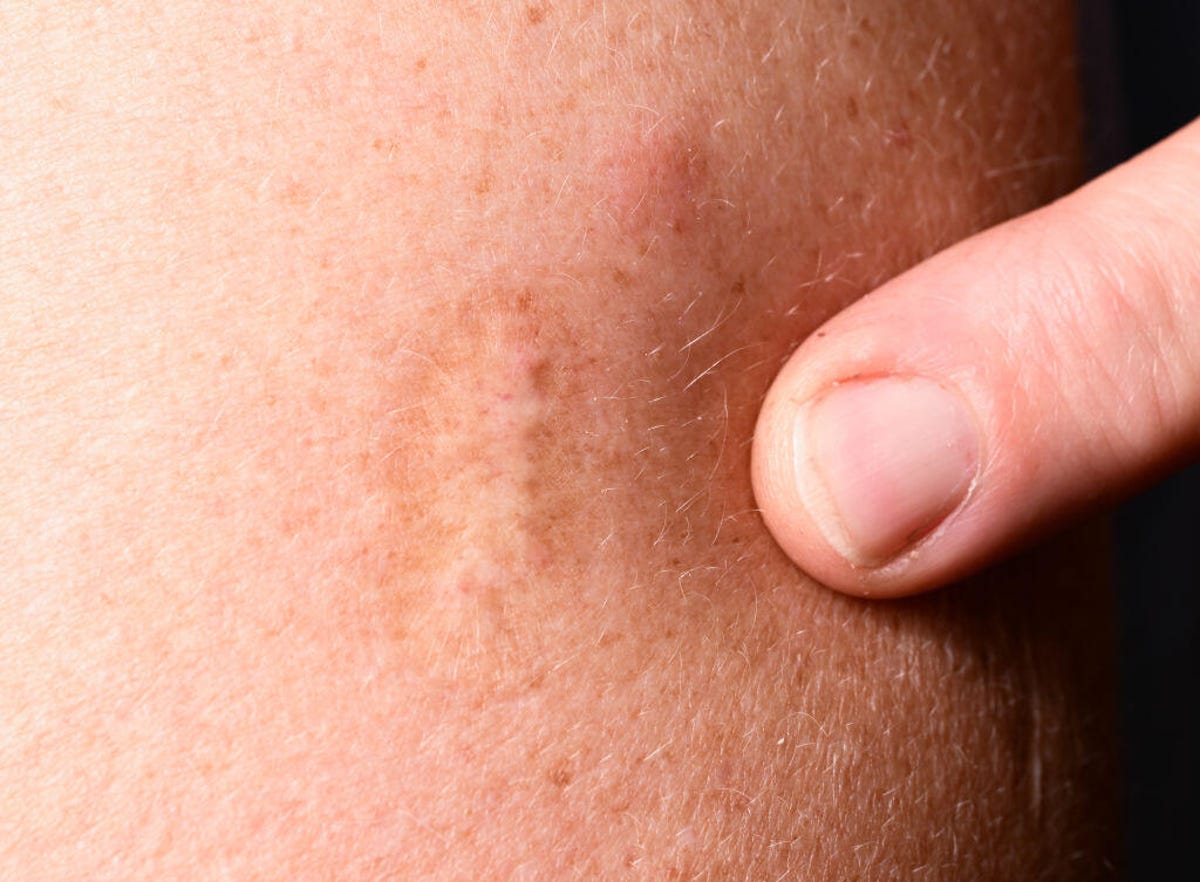

A smallpox vaccine scar. People born before the mid-1970s might have such a scar. Jynneos, a newer-generation monkeypox and smallpox vaccine, is not the same type of vaccine as the one used to eradicate smallpox and doesn’t leave a scar.
Picture Alliance/Getty ImagesWhat are the monkeypox vaccines? Who has immunity from the smallpox vaccine?
The US has two vaccines in its national stockpile that work against monkeypox. Jynneos is currently being given out to people before an exposure, as well as after an exposure, as it’s a newer vaccine that’s safe for most people.
Jynneos (made by Bavarian Nordic) is a new-generation vaccine approved by the US Food and Drug Administration in 2019 for monkeypox and smallpox. It’s a two-dose vaccine, with each shot given four weeks apart. It uses a weakened virus and is approved for adults 18 and older who are at high risk of getting monkeypox or smallpox.
Jynneos is the vaccine that is being shipped out and currently being used in the US monkeypox response. Because of limited supply, it’s likely you’ll only be able to book an appointment for the first dose for the time being. In New York City, for example, health officials say you’ll be contacted if you received a first dose about the second dose in the coming weeks.
ACAM2000 is a second-generation smallpox vaccine that also works against monkeypox. According to the CDC, ACAM2000 is a derivative of Dryvax, which helped eradicate smallpox. (The two diseases are closely related and both caused by orthopoxviruses, which makes this possible.) If necessary, the US Department of Health and Human Services said it’s also prepared to ship out the ACAM2000 vaccine, which is in greater supply than Jynneos but remains a second choice in the monkeypox response because it has a side effect profile that isn’t safe for certain people.
ACAM2000 is administered differently than the typical vaccine shot we’re used to, including intradermal injection. It’s given by dipping a needle into a vaccine solution which will then be “pricked” several times on the upper arm. It will cause a localized infection (a “pox”), prompting an immune response.
While ACAM2000 doesn’t cause smallpox, it contains live vaccina virus, which isn’t suitable for everyone. It could be unsafe for immunocompromised people, pregnant folks and those with certain heart or skin diseases, like eczema.
Smallpox was declared eliminated from the world in 1980. The US stopped routine vaccination against it in 1972, though some health care workers or people who work in labs may have had the vaccine. Historically, according to the CDC, smallpox vaccines were 95% effective against infection and protect you for about three to five years, and after that protection starts to wane.
Because of this, it’s possible people born before the early 1970s who got the smallpox vaccine might have some cross-protective immunity against monkeypox, according to the WHO, but there is “little immunity” to younger people living in non-endemic countries because they’ve had no exposure to a similar virus.
Read more: Monkeypox: What Gay and Bisexual Men Need to Know
How effective are the vaccines against monkeypox? How long do they take to work?
Giving Jynneos within four days of a monkeypox exposure is the best option for stopping the onset of the disease, according to the CDC. If it’s given four to 14 days after an exposure, the CDC says, Jynneos may not prevent monkeypox but will likely reduce the severity of symptoms. However, this information was published when Jynneos was given only the “standard” way (subcutaneous injection).
The CDC says that the Jynneos vaccine takes two weeks (14 days) after the second dose for immunity to build, and that ACAM2000 takes four weeks for maximum immunity. Though many people are receiving only the first dose of Jynneos at this time, early research suggests that one dose will still offer some protection, at least for a shorter period of time.
Because the US outbreak of monkeypox is so new, there’s no data yet on exactly how effective the vaccines will be in the current situation, according to the CDC. Effectiveness of Jynneos against monkeypox is supported by animal studies, the agency says. And though it won’t be used in most cases, ACAM2000 is closely related to Dryvax, the vaccine that helped get rid of smallpox. Effectiveness of ACAM2000 is supported by animal studies as well as human clinical trials.
You should still self-isolate if you develop symptoms of monkeypox after getting vaccinated, such as a rash.

A computer image of a monkeypox virus.
Uma Shankar Sharma/Getty ImagesWhy does the US have a stockpile of monkeypox vaccines?
The US has a stockpile of Jynneos and ACAM2000 on hand not because the country was worried about an outbreak of monkeypox (which has been endemic in some African countries for years), but in case smallpox becomes a public threat again. Smallpox was declared eliminated in the 1980s, and the last natural outbreak in the US happened in 1949. But smallpox is usually much more severe than monkeypox, and officials worry it could be used as biological warfare.
“The stockpile was created in the event of a biological weapons attack on the United States with smallpox,” Adalja says. In this event, health officials would not be rationing vaccines or using only the newer-generation vaccine, according to Adalja.
“If there was a smallpox attack, we would be using whatever vaccines we have to be able to deal with it,” he added.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.