What’s happening
Updated COVID boosters from Pfizer and Moderna are available to everyone age 5 and older. Some adults can get Novavax for a booster instead.
Why it matters
As the virus mutates, boosters have become necessary to restore some protection previously given by the original vaccines or past infections.
What’s next
OIder adults in their 60s and up, as well as other folks with medical conditions, continue to be at higher risk of severe COVID-19 disease. Boosters offer protection against severe disease, but other health measures like taking a rapid test before family gatherings can help protect the people around you.
Everyone age 5 and older is eligible for an updated booster, which is just one dose of either Pfizer’s or Moderna’s updated formulas, as long as two months have gone by since your last COVID-19 shot.
And for adults who can’t take an mRNA vaccine or just don’t want one, health officials have signed off on Novavax’s vaccine as a booster, as long as six months have gone by since you completed your primary vaccine (the first two doses, or one dose if you got Johnson & Johnson). This is for people who are fully vaccinated but haven’t gotten any booster yet, which accounts for about 48% of vaccinated adults in the US.
Updated or not, boosters have reduced the risk of hospitalization and death from COVID-19 across age groups, with people at higher risk of severe COVID-19 disease seeing large protective benefits. Unvaccinated adults who were 50 or older were 12 times more likely to die from COVID-19 than adults of the same age who had at least two booster doses, according to CDC data from April through early September. Adults of that age who weren’t vaccinated were three times more likely to die from COVID-19 compared with those who had one booster.
Compared with all adults 18 and older who were “up to date” on their COVID-19 vaccinations, meaning they got the shots they were eligible for, unvaccinated adults of the same age were more than five times more likely to be hospitalized.
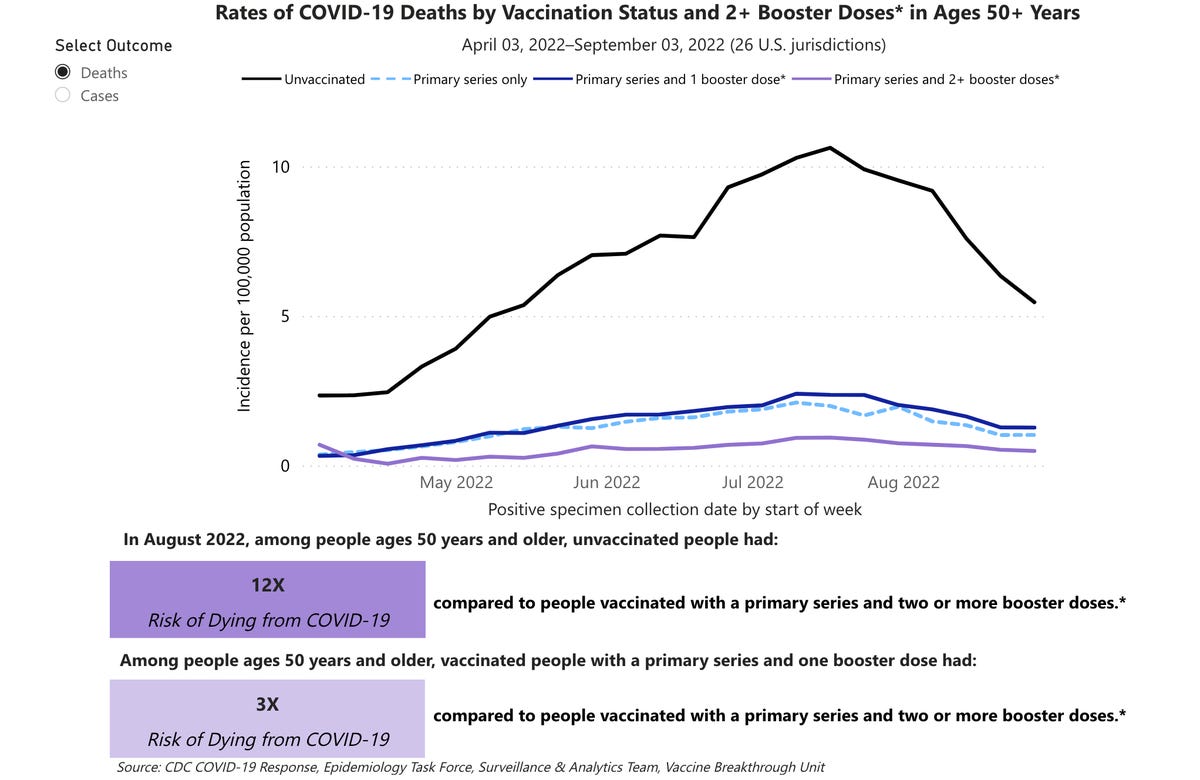

A graph showing risk of death from COVID-19 based on vaccination or booster status in adults 50 and older.
The US Centers for Disease Control and PreventionNot many people have gone in for a new booster yet, despite them being available since September. Only about 13% of adults ages have gotten a new dose, according to CDC data. About 30% of adults age 65 and up have gotten their new booster. While this is a better uptake than the younger age groups, older adults have the highest risk of getting really sick with or dying from COVID-19. More than 81% of all COVID-19 deaths have been in people over age 65, according to the CDC.
We’re officially in the holiday season, where many people will be gathering indoors with other people from all over. Taking a rapid test before hanging out with more vulnerable family members and staying home when you’re not feeling well remain protective health measures that can reduce the risk of disease to people around you, especially as many people may have an immunity gap from a distant vaccination or infection many months ago.
As one of the best protectors against severe disease, here’s what we know about the updated boosters, when you should get one and how they might fare against the growing BQ.1 and BQ.1.1 subvariants.

What are the new Pfizer and Moderna vaccines? Were they tested?
The new vaccines are bivalent, meaning they target the “original” strain of COVID-19, as well as the newer BA.4 and BA.5 subvariants that have made up most cases up until recently. Combined, BQ.1 and BQ.1.1 are now making up more cases, according to a CDC estimate.
Moderna announced positive results last week on its clinical trial with its new bivalent booster. In the largest trial to date on the updated bivalent vaccines, the company said that a booster dose of the new, bivalent vaccine “induced significantly higher neutralizing antibody titers against BA.4/BA.5,” compared with the original booster.
Pfizer and BioNTech earlier this month also announced promising results of its updated booster dose in older adults, reporting that adults older than 55 had a four times greater immune response compared with the original booster. The safety profile of Pfizer’s and Moderna’s new boosters were similar to their original vaccines, the companies said.
An early analysis also showed Moderna’s updated vaccine gave a “robust” antibody response against BQ.1.1, the company said — a subvariant of omicron which has now surpassed BA.5 — though less robustly when poised against BA.5.
The FDA has authorized two updated boosters from Pfizer-BioNTech and Moderna. Pfizer’s is for those age 5 and older, and Moderna’s is for people 6 and up.
When they were authorized by the FDA, the agency made its decision based on clinical trials of a slightly different bivalent booster that targets the BA.1 version of omicron instead of BA.4/BA/5. In addition, there were mice studies on the BA.4/BA.5 formula. While this approach was previously unprecedented in the COVID-19 vaccine campaign, health officials say it’s not too far off the way we approve influenza vaccines. Every year, the flu vaccine is tweaked with a strain believed to be the best target. And the new COVID-19 boosters also do not introduce any new vaccine ingredients.
“Bivalent and multivalent vaccines are very common and modifying a vaccine to include different virus strains often does not require a change in other ingredients,” FDA Commissioner Dr. Robert M. Califf said in August in a tweet. “FDA has extensive experience with reviewing strain changes in vaccines, as is done with the annual flu vaccine.”
But in November, Moderna and Pfizer announced results (not yet peer reviewed) from their human trials on the new vaccines, showing that they appear to produce a stronger antibody response than the original vaccines. This is welcome news, as two earlier, preliminary studies had suggested the updated formulas were about as protective, or only slightly better, than the original COVID-19 vaccine formulas.
Who can get Novavax’s booster?
Adults who’ve been vaccinated against COVID-19 — but haven’t yet received any booster — can get a dose of Novavax as their booster. It doesn’t matter which vaccine you originally received — Pfizer, Moderna, Johnson & Johnson or Novavax.
Read more about the Novavax vaccine here.
What about BQ.1?
BQ.1 and BQ.1.1 are now the most common subvariants causing COVID-19 infections, accounting for about 44% combined. BA.5 is causing about 30% of current COVID-19 cases, according to the CDC’s estimates.
Like BA.5, the “Q” subvariants are still considered versions of omicron, and neither have been given a separate “variant of concern” label — a separate designation researchers give to variants that have become significantly more contagious, severe or cause public health tools, like vaccines or antivirals, to be significantly less effective.
While scientists were expecting new mutations as the virus continues to spread and evolve, it might be too early to tell how the newer versions of COVID-19 will play out in the US. Moderna said Monday that its early analysis showed that its bivalent vaccine did produce an antibody response against BQ.1.1, though a weaker response compared to BA.5.
Dr. Jayne Morgan is the executive director of the COVID Task Force at Piedmont Healthcare and also has a YouTube channel dedicated to “demystifying” science and medicine. She said that by allowing COVID-19 infections to occur, we are allowing the virus an opportunity to invade our bodies and mutate into new variants, many of which have been more contagious than previous versions and caused more hospitalizations and deaths.
“Whether they turn into something or nothing, the fact of the matter is they continue to give us evidence that these variants will continue to develop if we continue to make ourselves available to be infected,” Morgan said. “Human beings are the key part of the life cycle of this virus.”
Boosters and public health measures like wearing masks are some ways we can stop participating in the life cycle of the virus and help it die down, Morgan says.
When should I get another booster?
The updated Moderna and Pfizer-BioNTech boosters are authorized by the FDA for people who’ve gone at least two months since their last vaccine dose, whether it was a booster shot or a primary series. It doesn’t matter which vaccine you originally received, and it shouldn’t matter which brand you choose now. Moderna’s new booster, like its previous vaccine, is a slightly larger dose (50 micrograms) than Pfizer’s (30 micrograms).
If you were vaccinated earlier in the pandemic but haven’t gotten any booster yet, you can get a Novavax shot at least six months after finishing your primary series.
At a panel meeting of the CDC’s scientific advisers, a committee that meets before the CDC recommends a vaccine, a few members expressed concern that some people would be better off waiting longer than two months between their last shot and this new booster, especially people who have recently had COVID-19 and still have relatively high immunity. (In its general vaccine guidance, the CDC says that people can wait three months before getting the shot if they’re getting over COVID-19.)
And while myocarditis is rare overall, younger men and teenage boys appear to be at higher risk post-vaccination and can wait longer between vaccine doses appears to reduce this risk.
From an immune response perspective, some infectious disease doctors have suggested waiting as long as four to six months between your last COVID-19 infection or vaccine to get the most bang out of the new booster. Dr. Anthony Fauci , the chief medical adviser to the president, told PBS that if you tested positive for COVID-19 recently, “you should wait about three months, at least three months from the time that you had a prior infection” before getting the new booster. Fauci added that because he had COVID-19 in the middle and end of June, he was waiting until the end of September to get boosted.
Fauci ended up getting his booster live on The Late Show With Stephen Colbert in early October.
But individual immunity is only one piece of the puzzle, when we’re talking about a respiratory virus as contagious as COVID-19. People who may only have very mild symptoms they dismiss as a cold or allergies can still pass along an infection to their more-vulnerable family or friends. And as people make their Thanksgiving plans and the New Year inches closer, this is becoming an especially important element to consider.
“Even if you yourself are on the low-risk side, you’re going to have family and friends you’re going to see,” Dr. Ashish Jha, the White House COVID-19 Response Team Coordinator said on Andy Slavitt’s podcast in October, as reported by CNBC.
“You don’t want to be the person who gives it to your grandma.”
Morgan said that it’s important to get a booster right now so we can “stem the tide” on the pandemic.
“You want to have this booster while omicron is circulating. The whole point of bringing the booster out now is so that we can stop chasing this variant,” Morgan said, adding that it’s unclear what variants or subvariants will be in our future, and if (or how much) they’ll evade our protection from vaccines, infections and treatments.
The vaccines and boosters have proven to be especially effective at preventing severe disease in older adults. A report published Oct. 7 by the US Department of Health and Human Services found that COVID-19 vaccines were linked to about 650,000 fewer hospitalizations and 300,000 fewer deaths in seniors and other people who are enrolled in Medicare in 2021. More than 81% of COVID-19 deaths occur in people over age 65, according to the CDC.
Where can I get the new booster?
You should be able to use the vaccine finder site Vaccines.gov to find an updated vaccine near you. When you’re choosing your updated booster from either Pfizer-BioNTech or Moderna, make sure to select the shot that says “Newly Authorized Bivalent” in bold text. Primary series vaccines, or the first two doses, are still available for people who haven’t received any vaccine yet.
Smaller doses of the original vaccines are also still available for kids as young as six months. For those who are getting the Novavax booster, you’ll find it under “Primary vaccines.”
Can I get a flu vaccine at the same time as my booster?
Yes, according to the CDC. There’s no recommended waiting period between the seasonal flu shot and the COVID-19 vaccines.
The flu vaccines for the 2022-2023 season have been updated, and the CDC considers September or October good months for most people to get their flu shot.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.